Abstract
Introduction: MYD88 L265P mutations have been described in more than 90% of Waldenström's Macroglobulinemia (WM) patients. MYD88 wild-type (wt) WM represent less than 10% of WM, and associate with shorter overall survival versus MYD88 mutated (mut) WM, as well as with lack of major responses and inferior progression-free survival to ibrutinib. Mutated MYD88 triggers WM cells growth and survival through NF-kB activation via IRAK1/4, BTK and HCK transactivation. CXCR4 mutations promote pro-survival AKT and ERK1/2 signaling and are associated with delayed response to ibrutinib. Deletions involving the long arm of chromosome 6 (Del6q) are highly recurrent in various B-cell malignancies, particularly those that are driven by mutated MYD88, and have been observed in up to half of WM patients. Chr6q genomic loss in WM affect important modulators of NFkB (TNFAIP3, HIVEP2), BCL2 protein family (BCLAF1), apoptosis and IGF1/PI3K/AKT signaling (FOXO3), and BTK (IBTK). The frequency and impact of Del6q on the different patient genotype remains to be clarified.
Patients and Methods: Based on location and known regulation of key pathways in WM, copy number alterations (CNA) and gene expression level were assessed for IBTK, FOXO3, BCLAF1, TNFAIP3 and HIVEP2 genes, along with CXCR4 transcriptional levels. DNA and RNA from CD19+ sorted BM lymphoplasmacytic cells from 32 untreated WM and one IgM and IgG-secreting lymphoplasmacytic lymphoma patients were analyzed. Paired CD19-depleted peripheral-blood mononuclear cells (PBMCs) were used as germline controls. Paired CD19+ and CD19- PBMCs from 6 healthy donors were analyzed to rule out possible B-cell specific findings. CNA were measured in quadruplicate and gene expression in triplicate using TaqMan real-time polymerase chain reaction (RT-PCR) assays. Deletions affecting less than 20% of WM cells were considered to be beneath the RT-PCR detection threshold. The cohort included 33 patients (21 males, 12 females), with a median age of 62 (range 35-91) years, BM involvement of 60% (range 2.5-90%), serum IgM levels of 3010 (range 257-6910) mg/dl, and hemoglobin of 10.9 (range 8.4-14.4) g/dl. MYD88 L265P mutations were detectable in 25 (76%) patients, 11 of whom (44%) also carried CXCR4WHIM mutations. Eight patients (24%) were MYD88 and CXCR4 wt.
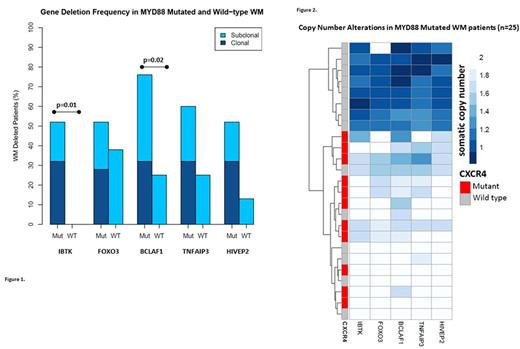
Results: Somatic Del6q were observed in 4/8 (50%) and in 20/25 (80%) patients in the MYD88 wt and mut population, respectively (p=NS). In contrast, no CNA were present in the HDs CD19+/CD19- compartments. IBTK remained intact in all 8 MYD88 wt WM, whereas resulted deleted in 13/25 (52%) MYD88 mut patients (p=0.01). BCLAF1 deletion frequency was significantly different among the two subgroups, with only 2/8 deleted MYD88wt (25%) and 19/25 (76%) deleted MYD88 mut patients (p=0.02; Figure 1). Eight MYD88 mut WM (32%) showed contiguous fully clonal Del6q, that spanned all studied genes in 7/8 (87.5%) cases; in contrast, contiguous subclonal deletions occurred only in 3/12 (25%) cases (p=0.02). All 8 fully clonal Del6q patients were CXCR4 wt; in contrast, CXCR4 mut were detected in 9/12 (75%) patients with subclonal Del6q (p=0.001; Figure 2). Unlike the MYD88 mut population, all 4 MYD88 wt patients presented with non-contiguous subclonal deletions (p=NS). Fully clonal deletions of IBTK, BCLAF1 and HIVEP2 significantly reduced the respective gene transcriptional levels (p= 0.03, 0.01 and 0.01, respectively), while CNA in TNFAIP3 and FOXO3 did not affect their expression. The MYD88 wt patients showed significant decreases in the transcriptional levels of BCLAF1 related to gene subclonal deletions, and reduced CXCR4 levels compared to MYD88 mut WM (p=0.02 and p=0.02, respectively).
Conclusions: Our findings demonstrate that gene loss of IBTK, FOXO3, BCLAF1, TNFAIP3 and HIVEP2 occurs in most WM patients, but at a lower frequency in MYD88 wt. Unlike the MYD88 mut patients, those MYD88 wt showed no contiguous fully clonal deletions. The latter correlate with CXCR4 wt and reduce only IBTK, BCLAF1 and HIVEP2 levels, suggesting that regulatory mechanisms may compensate for FOXO3 and TNFAIP3 gene loss. Significant variation in IBTK and BCLAF1 deletion rate suggest that BCR and BCL2 signaling may play a different role in MYD88 mut and wt WM. The findings provide valuable insights into MYD88 wt WM pathogenesis, and may be relevant to understanding therapeutic outcome with agents that target MYD88, CXCR4, BTK and BCL2.
Castillo: Millennium: Research Funding; Abbvie: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Arcaini: Pfizer, Celgene, Bayer, Roche: Membership on an entity's Board of Directors or advisory committees; Celgene, Roche, Sandoz: Consultancy; Gilead: Research Funding. Varettoni: Jannsen: Consultancy, Other: travel expenses. Treon: Pharmacyclics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal